Medication (e.g., minoxidil, finasteride)1,2, hair transplantations (e.g., strip harvesting, FUE)3 and concealers (e.g., scalp tattooing, hair thickening fibers)4 are among the many hair loss treatments available today. If you prefer a more non-invasive treatment that doesn’t involve the use of medication, low level laser therapy (LLLT) could be the hair loss treatment for you.
The development of LLLT can be traced back to the 1960s. Dr. Mester was one of the first to observe the effects of light therapy (or laser therapy) on hair loss. Using a laser that emitted a ruby color beam (694 nm), Dr. Mester noticed rapid hair regrowth in mice5. Since this discovery, several studies on the effects of laser therapy on humans have taken place.
How Does Low Level Laser Therapy Work?
Lasers can be used to create changes in human cells. In the case of low level laser therapy (LLLT), lasers can be used to promote hair growth6. Lasers are made up of three main ingredients; an active material (a solid, liquid or gas), two mirrors and a light source that excites the active material7. When a laser is used in LLLT, light is emitted onto the skin and is absorbed by specific parts of the human body called chromophores or photo-acceptors. During LLLT, common chromophores found in the skin (e.g., hemoglobin, melanin and water) absorb very little emitted light, allowing this light to be absorbed by other key photo-acceptors associated with hair growth6. It is thought that light absorption by the photo-acceptor cytochrome c oxidase can help stimulate hair growth through prolonging the hair growth phase and preventing hairs from ending their growth phase prematurely8. The exact process of how LLLT is able to promote hair growth is not yet fully understood and research is still being conducted to better understand this process8.
Despite not knowing the exact details, we do know that the effectiveness of LLLT can depend on the wavelength used. During LLLT, wavelengths within the visible light spectrum are used to treat hair loss9. Specific wavelengths, such as those associated with red light (650 nm), have shown to be quite effective when used with LLLT10,11. Power could also contribute to the effectiveness of LLLT. LLLT is delivered in a low power setting as higher power can result in first or second degree burns6. Due to the low power setting used, LLLT is safe and doesn’t require the use of any protective equipment (e.g., goggles) during treatment.
In addition, treatment duration can also impact results. The treatment length and time between treatments can influence success as higher dosages of light may not be as beneficial as lower dosages of light9. LLLT treatments need to occur on a regular basis for an extended period of time, approximately 6 months, in order to see results.
The effectiveness of LLLT also depends on the presence of follicles as follicles are needed for the stimulating effect of LLLT to occur. The sooner you try LLLT the better. Patients with thinning hair have a greater chance of obtaining desired results as opposed to those that have been bald for quite some time.
What are my LLLT Options?
In 2007, the first LLLT device, the HairMax LaserComb, was approved by the FDA12. This device was approved to encourage hair growth in male androgenic alopecia patients13. The comb induces hair growth by penetrating light into hair follicles through the comb’s teeth9,14. In a randomized double-blind trial, the HairMax LaserComb was able to increase hair count and increase thickness and fullness of hair after 26 weeks of treatment15.
In addition to the HairMax LaserComb, The Hair Growth Stimulation System (a non-heating lamp) and the iGrow-II Hair Growth System (a ‘laser cap’) are other approved LLLT options available16,17. Incorporating LLLT into a helmet or cap, as in the case of the iGrow-II Hair Growth System, LLLT can become more user-friendly. Through the use of a helmet apparatus, promising results were found in both male and female patients; with a 35% to 37% increase in hair growth after 16 weeks of treatment10,11.
Prescriptions are not needed to obtain any of these FDA approved devices. You can purchase LLLT devices online or through a physician’s office. If you don’t want to invest in an LLLT device, there are many clinics that offer LLLT treatments such as Sure Hair International London. If you aren’t entirely convinced that LLLT is effective, the following before and after pictures of patients undergoing LLLT might ease your mind.
LLLT Testimonials
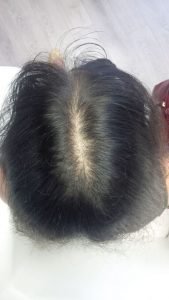
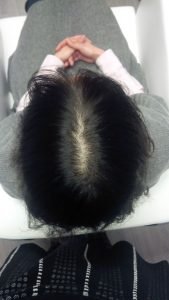
Patient 1: This patient underwent 4.5 months of therapy, receiving LLLT treatment once a week.
Before LLLT: After LLLT:
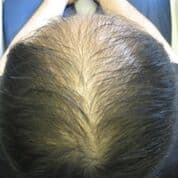
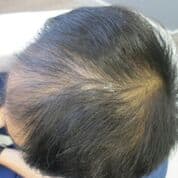
Patient 2: This patient underwent 4 months of therapy, receiving LLLT treatment once a week.
Before LLLT: After LLLT:
If you would like to know more about LLLT be sure to ask a Sure Hair representative.
Article by: Sarah Versteeg MSc, Mediprobe Research Inc.
References
- Product Monograph. Hair Regrowth Forumula. Minoxidil Topical Solution USP 20 mg/mL (2% w/v) [Internet]. Health Canada. Drug Product Database. 2016 [cited 2016 Oct 6]. Available from: file:///C:/Users/sversteeg/Downloads/PM00033538.PDF
- PROPECIA® (finasteride) tablets for oral use [Internet]. FDA U.S. Food and Drug Administration. 2014 [cited 2016 Nov 8]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/020788s024lbl.pdf
- Gho CG, Neumann HAM. Advances in Hair Transplantation: Longitudinal Partial Follicular Unit Transplantation. Curr Probl Dermatol. 2015 Feb;47:150–7.
- Harris AG, Kim M, Murrell DF. Pigmented Hair-Thickening Fibers: A Camouflage Technique for Alopecia in Patients with Epidermolysis Bullosa. Skin Appendage Disord. 2016 Feb;1(3):153–5.
- Mester E, Szende B, Gärtner P. [The effect of laser beams on the growth of hair in mice]. Radiobiol Radiother (Berl). 1968;9(5):621–6.
- Keene SA. The science of light biostimulation and low level laser therapy (LLLT). Hair Transpl Forum Int. 2014 Dec;24(6):201,208–9.
- BM Biomedic. 2012 [cited 2016 Dec 13]. Available from: https://biomediclaser.com/biomediclaser.org/files/LASER_P1.pdf
- Avci P, Gupta GK, Clark J, Wikonkal N, Hamblin MR. Low-level laser (light) therapy (LLLT) for treatment of hair loss. Lasers Surg Med. 2014 Feb;46(2):144–51.
- Chung H. The Nuts and Bolts of Low-level Laser (Light) Therapy. Ann Biomed Eng. 2012 Feb 1;40(2):516–33.
- Lanzafame RJ, Blanche RR, Bodian AB, Chiacchierini RP, Fernandez-Obregon A, Kazmirek ER. The growth of human scalp hair mediated by visible red light laser and LED sources in males. Lasers Surg Med. 2013 Oct;45(8):487–95.
- Lanzafame RJ, Blanche RR, Chiacchierini RP, Kazmirek ER, Sklar JA. The growth of human scalp hair in females using visible red light laser and LED sources. Lasers Surg Med. 2014 Oct;46(8):601–7.
- 510(k) Premarket notification – Laser, Comb, Hair [Internet]. U.S. Food and Drug Administration. 2016 [cited 2016 Dec 13]. Available from: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?id=K060305
- Product Classification – Laser, Comb, Hair [Internet]. U.S. Food and Drug Administration. 2016 [cited 2016 Dec 13]. Available from: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpcd/classification.cfm?ID=5180
- Leavitt M, Charles G, Heyman E, Michaels D. HairMax LaserComb® Laser Phototherapy Device in the Treatment of Male Androgenetic Alopecia: A Randomized, Double-Blind, Sham Device-Controlled, Multicentre Trial. Clin Drug Investig. 2009;29(5):283–92.
- Jimenez JJ, Wikramanayake TC, Bergfeld W, Hordinsky M, Hickman JG, Hamblin MR, et al. Efficacy and Safety of a Low-level Laser Device in the Treatment of Male and Female Pattern Hair Loss: A Multicenter, Randomized, Sham Device-controlled, Double-blind Study. Am J Clin Dermatol. 2014;15:115–27.
- 510(k) Summary – MEP-90 Hair Growth Stimulation System [Internet]. FDA U.S. Department of Health & Human Services. 2009 [cited 2016 Dec 13]. Available from: http://www.fda.gov/cdrh/510k/K091496.pdf
- K10K Summary – Apira-Science, Inc. [Internet]. FDA U.S. Food and Drug Administration. 2012 [cited 2016 Dec 13]. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf12/K122248.pdf