Propecia – Information Fact Sheet
Finasteride 1 mg
What is it?
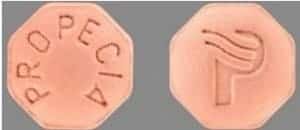
Propecia is a film-coated tablet that contains finasteride, a medicinal ingredient used to treat hair loss. These tablets are tan in color and are easy to identify (Figure 1).
Figure 1. Example of Propecia tablets
Health Canada Approval
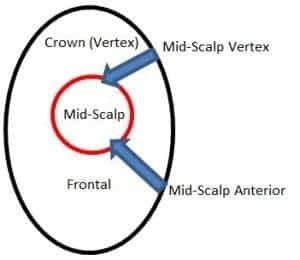
Finasteride 1 mg (Propecia) is Health Canada approved to treat men with mild to moderate scalp hair loss located in the vertex and anterior mid-scalp regions (Figure 2). Propecia is not approved to be used in women or children. Finasteride 5 mg (Proscar) is approved for benign prostatic hyperplasia and for the prevention of urologic events (e.g. acute urinary retention etc.).
Figure 2. Propecia is approved to treat men with mild to moderate scalp hair loss located in the vertex and anterior mid-scalp regions (blue arrows).
Dose
Propecia (1 mg tablet) should be taken once a day. Long term use is suggested by Health Canada as improvement in hair restoration parameters (e.g., hair regrowth) are projected to occur several months after use. It is recommended that patients do not discontinue treatment when desired results are achieved as hair growth gained during treatment could be lost. Extra tablets should not be taken if a dose or multiple doses are missed. The bioavailability of Propecia is not impacted by food, thus Propecia can be taken with or without food.
How does it work?
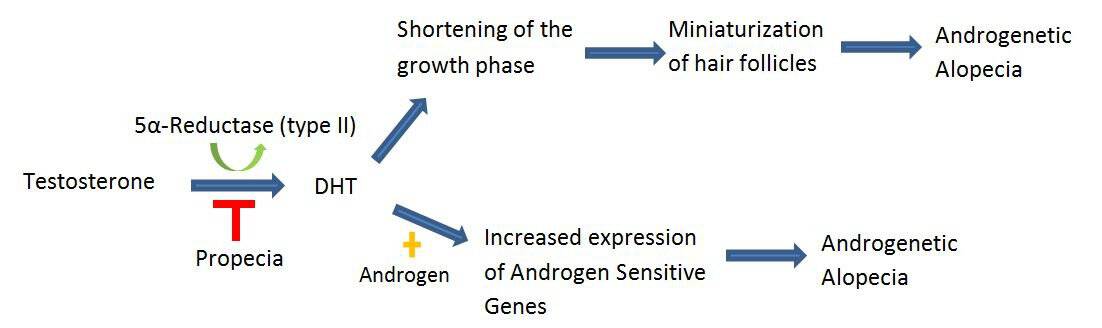
Finasteride competitively inhibits type II 5a-Reductase. 5a-Reductase catalyzes the conversion of testosterone to 5a-Dihydrotestosterone (DHT) (Figure 3). Increased DHT levels can shorten follicular growth resulting in the creation of miniaturized hair follicles (Figure 3). DHT can also contribute to hair loss through increasing the expression of androgen sensitive genes (Figure 3). Drugs which have a high affinity for type II 5a-Reductase, such as finasteride, inhibit the creation of DHT thus preventing the progression of hair loss, encouraging hair regrowth.
Figure 3. Finasteride competitively inhibits type II 5a-reductase, preventing the progression of hair loss and encouraging hair regrowth.
Stabilization of hair loss and increased hair counts have been reported to occur in Propecia treated men. These results were observed as early as 3 months into treatment. An improvement in hair growth was found in 65% of treated men with no improvement in hair growth reported in placebo treated men. Propecia can also improve hair appearance in men with frontal/mid-area hair loss as evident in a 12 month study. Anagen (growth phase) to telogen (rest phase) ratios can be improved using Propecia as found in a 48 week placebo controlled study using androgenetic alopecia (AGA) patients, confirming that Propecia can encourage hair regrowth.
Propecia is not effective in postmenopausal AGA women, showing no improvement in hair restoration parameters (e.g. hair count) in a 12 month placebo control study.
Warnings and Precautions
As previously mentioned, Propecia is not indicated for women or children. Pregnant women should not handle crushed or broken tablets as Propecia could impact male fetuses if topically absorbed. Clinical studies with Propecia have been directed towards middle aged men thus more research is required before Propecia should be prescribed to male pattern hair loss patients over the age of 65. In some countries, such as South Korea, the warning label on Propecia has been altered to include depression and suicidal ideation. If you experience these symptoms you should stop taking Propecia and consult your physician for alternative treatment options.
Contraindications
Propecia is contraindicated in women who are pregnancy or may become pregnant and those that have a hypersensitivity to the product’s medicinal and nonmedicinal ingredients. The nonmedicinal ingredients contained in Propecia 1 mg tablets include; docusate sodium, hydroxypropylcellulose, lactose monohydrate, magnesium stearate, methylhydroxypropylcellulose, microcrystalline cellulose, pregelatinized starch, sodium starch glycolate, talc, titanium dioxide, red ferric oxide and yellow ferric oxide. To avoid an adverse reaction, please inform your doctor of any allergies you may have.
Drug-Drug Interactions
The product insert for both Proscar and Propecia state that, “no drug interactions of clinical importance have been identified”. Clinical studies suggest that concomitant administration of finasteride with angiotensin-converting enzyme inhibitors, anticonvulsants, benzodiazepines, beta blockers, calcium-channel blockers, cardiac nitrates, diuretics, H2 antagonists, HMG-CoA reductase inhibitors, prostaglandin synthetase inhibitors and quinolone anti-infectives, do not result in significant adverse interactions. As a precautionary measure, be sure to inform your doctor of any changes in your health or additional medications you might be taking.
Side effects
Health Canada and the Food and Drug Administration (FDA) consider Propecia as a safe and effective means to treat hair loss.
Patients using Propecia should inform their doctor when undergoing prostate cancer screening as finasteride can impact prostate tissue, decreasing prostate specific antigen (PSA) levels by approximately 40%. There is some evidence to suggest that 5a-Reductase inhibitors, such as finasteride 5mg, could increase the risk of prostate cancer (finasteride 1.8% vs placebo 1.1%). However these results were obtained at a finasteride concentration 5 times that of Propecia.
Adverse events due to Propecia are uncommon however sexual side effects such as a decreased libido, erectile dysfunction, and ejaculation issues (e.g. decreased volume of ejaculate) have been reported. These sexual side effects approximately affect 1 in every 100 Propecia treated patients. Cessation of treatment commonly resolves sexual side effects; however in a small percentage of patients’ effects have continued post treatment. This condition is commonly referred to as Permanent Erectile Dysfunction Syndrome. Other infrequently reported adverse events include, breast enlargement, allergic reactions, and testicular pain.
Contact your doctor if you are experiencing any of these aforementioned adverse events.
Want more information?
We recommend speaking with your doctor if you have questions on the use of Propecia for the treatment of hair loss.
Product monographs can also be a great source of information and were used to create this fact sheet. Product monographs for both Propecia and Proscar are freely accessible through Health Canada’s Drug Product Database at the following website:https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/drug-product-database.html.